
Weathering rates for mined lands exponentially higher than unmined sites
Mountaintop removal, a coal-mining technique used in much of Central Appalachia, is an extreme form of surface mining, that excavates ridges as deep as 600 feet — twice the length of a football field — and buries adjacent valleys and streams in bedrock and coal residue. This mining activity has long been known to have negative impacts on water quality downstream.
A new study led by watershed scientist Matthew Ross at Colorado State University found that many of these water quality impacts are caused by a dramatic increase in the chemical weathering rates of mined landscapes, which are melting away bedrock up to 45 times faster than unmined areas. In addition, the weathering has global consequences for the cycling of sulfur, which is a key nutrient for all life forms.

The findings show that when people move large quantities of bedrock and soil to build cities or to extract resources, they can completely alter and accelerate the natural weathering processes on land, which can impact water quality downstream.
Ross, an assistant professor in the Department of Ecosystem Science and Sustainability, described the chemical weathering rates as one of the highest rates ever observed, when compared to landscapes across the globe.
The study, “Pyrite oxidation drives exceptionally high weathering rates and geologic CO2 release in mountaintop-mined landscapes,” was published in the journal Global Biogeochemical Cycles.
Carbon cycle disrupted
This increased weathering — like many mine-related impacts — starts when iron sulfide or pyrite, a mineral also known as fool’s gold often found in coal, is exposed to air. This creates sulfuric acid, making water draining from the mine extremely acidic and caustic. To neutralize the acid, in much of Central Appalachia the pyrite-bearing rock is intentionally surrounded by and mixed with carbonate rocks.
While this limits acid-mine drainage problems, these acid-producing and -neutralizing reactions create ideal conditions for rapid chemical weathering of bedrock, with surprising implications for geologic carbon cycling of these landscapes.
In most areas that experience chemical weathering, carbon dioxide dissolves into carbonic acid, a weak weathering agent. When carbonic acid reacts with silicates or rock-forming minerals, carbon dioxide is permanently locked into the bedrock, balancing the carbon cycle over millions of years. In unmined landscapes, this process provides a slow but inevitable sink for atmospheric carbon dioxide, or CO2.
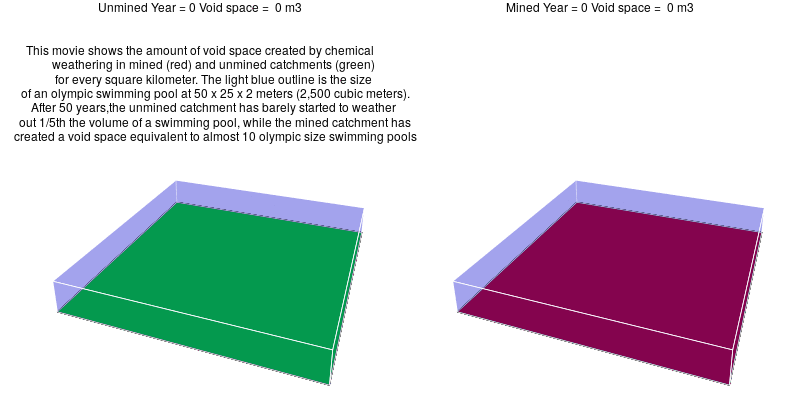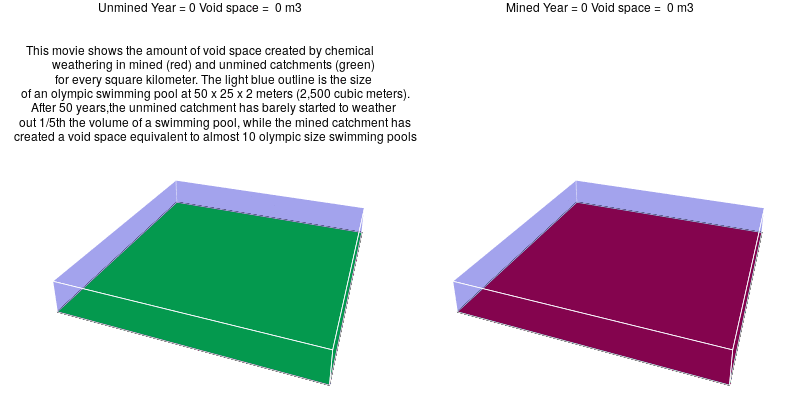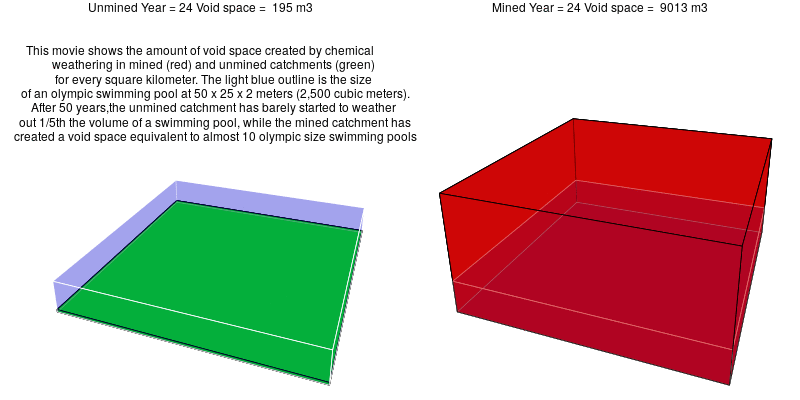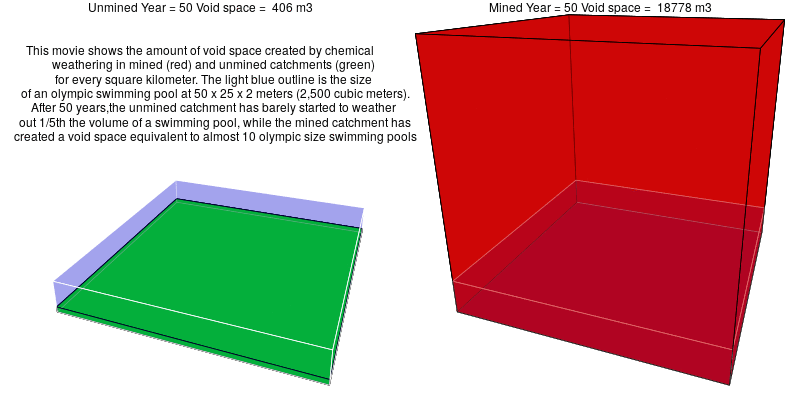
In mined landscapes with abundant sulfuric acid, the weathering reactions no longer rely on carbonic acid, and the potential for geologic carbon sequestration is eliminated. Instead, the sulfuric acid weathers out acid-neutralizing carbonates, which releases carbon dioxide into the atmosphere.
This means that long after mining in these areas has stopped, researchers estimate that between 20 percent and 90 percent of the carbon absorbed by plants on the surface will be cancelled out by the release of rock carbon to the atmosphere.
“Because this weathering is happening so fast and it is powered by sulfuric acid, it creates a landscape that is a source for carbon dioxide,” Ross said. “You’re rapidly dissolving away the landscape and releasing a bunch of rock carbon.”
This regional impact also has global consequences for the cycling of sulfur, an element that is important for all life forms. While mountaintop mining operations in Appalachia cover a small portion, .006 percent, of the land area on Earth, they may contribute as much as 7 percent of the total global delivery of sulfur from land to ocean.
This research, funded by the National Science Foundation, is part of an ongoing project led by Ross, who recently joined the faculty at the Warner College of Natural Resources.
Study co-authors include Fabian Nippgen, Department of Ecosystem Science and Management at the University of Wyoming, and Duke University’s Brooke Hassett, Brian McGlynn, and Emily Bernhardt.
The article was written by Mary Guiden and originally appeared in the WCNR news.